Hi Iseki,Thanks, FF, we are all in the same boat. Medical ethics is a minefield. I don't want to buy shares in a company that sells hope by supplying drugs to patients who the FDA consider to be so hopeless, all other treatments having failed, that they can go onto the company's clinical trial. ie read the special conditions of each clinical trial and decide for yourself what the FDA is telling you.
I look forward to the increased use of AI, including Akida, to help design the new drugs and treatments, that aren't just magic bullets shot into the dark.
I'm sure you are not advocating throwing the baby out with the bath water.
There are many cancer drug research companies which perfrom ethical research, provided your philosophy allows mouse tests.
One particularly promising avenue of research is the use of cancer antigens to specifically target cancerous cells.
One such Australian company has developed such an antigen which, on its own, can inhibit the reproduction of cancer cells by penetrating the cell membrane and interrupting the cell's DNA damage repair mechanism. The antibody has the ability to cross the blood-brain barrier to attack difficult to treat glioblastomas. In addition, the antibody can act as a vector to carry anticancer drugs and radiation targets specifically into cancer cells.
These characteristics mean that the use of these antibodies has the potential to signiicantly reduce the adverse side effects of chemotherapy and reduce the collateral damage caused by conventional chemotherapy and radiation treatment.
Just as with COVID or any other drug, it is necessary that the drugs undergo clinical trials on human volunteers. Once the drugs have been proved to be safe and effective on lab mice, the drugs need to be tested on healthy subjects to determine the degree of toxicity, and then they need to be tested on cancer patients to determine their effectiveness.
The drug development companies need to have insurance, and the insurance companies will, as is their nature, seek to limit their potential liability. I have not read the FDA conditions, but I imagine the insurance liability is, at least in part, responsible for some of the exclusions.
There is hope for effective treatment in the foreseeable future.
Hey Diogenese (aka Barrel),
I was just looking at this tutorial at the May 2019 Embedded Vision Summit. Yoshio Sato, Senior Product Marketing Manager in the Industrial Business Unit at Renesas, says at the 22 minute mark that they are looking for partners to work with Renasas on the Roadmap for DRP to enhance embedded AI inference capabilities.
I guess this is where BrainChip fits in, or could fit in.
View attachment 1147
Hi Bravo,
Well possibly that's where the BrainChip/Renesas hook-up started.
I would think that incorporating Akida in the Renesas DRP would have involved a major redesign of their DRP.
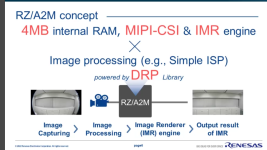
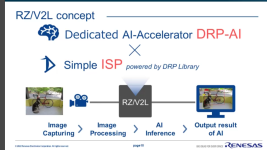
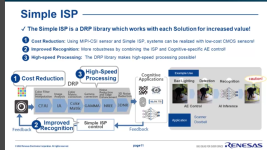
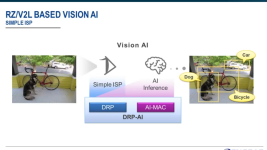
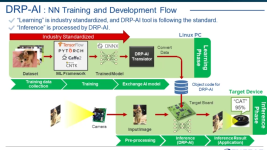
This is from my files, but I don't have a link, but you should be able to find it on the Renesas site: