D
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
BRN - NASA
- Thread starter TechGirl
- Start date
Fact Finder
Top 20
Hi Rocket
Already seen and read this one did nothing for me particularly. FF
Fact Finder
Top 20
Hi Rocket
Interesting read and shows what scope there is for AKIDA technology in its current and future iterations. Its ability to process all the five sensors will be incredibly valuable for providing the type of safe work place astronauts require if the Moon and Mars are to become viable destinations. If anyone has the inclination the problem on the Russian space station last year requiring emergency action by the NASA astronauts to survive was a classic example of why latency is a big no, no when monitoring in space.
My opinion only DYOR
FF
AKIDA BALLISTA
D
Deleted member 118
Guest
Attachments
D
Deleted member 118
Guest
Attachments
D
Deleted member 118
Guest
Attachments
D
Deleted member 118
Guest
Probably not what I thought they might be
D
Deleted member 118
Guest
Been trying to find any link between this company with 3 employees and Akida, but so far nothing.
 www.exploration.institute
www.exploration.institute
 www.sbir.gov
www.sbir.gov
Exploration Institute Homepage, Welcome.
EXPLORATION INSTITUTE LLC | SBIR.gov
Attachments
Last edited by a moderator:
D
Deleted member 118
Guest
D
Deleted member 118
Guest
D
Deleted member 118
Guest
Can’t remember if I’ve posted this one before
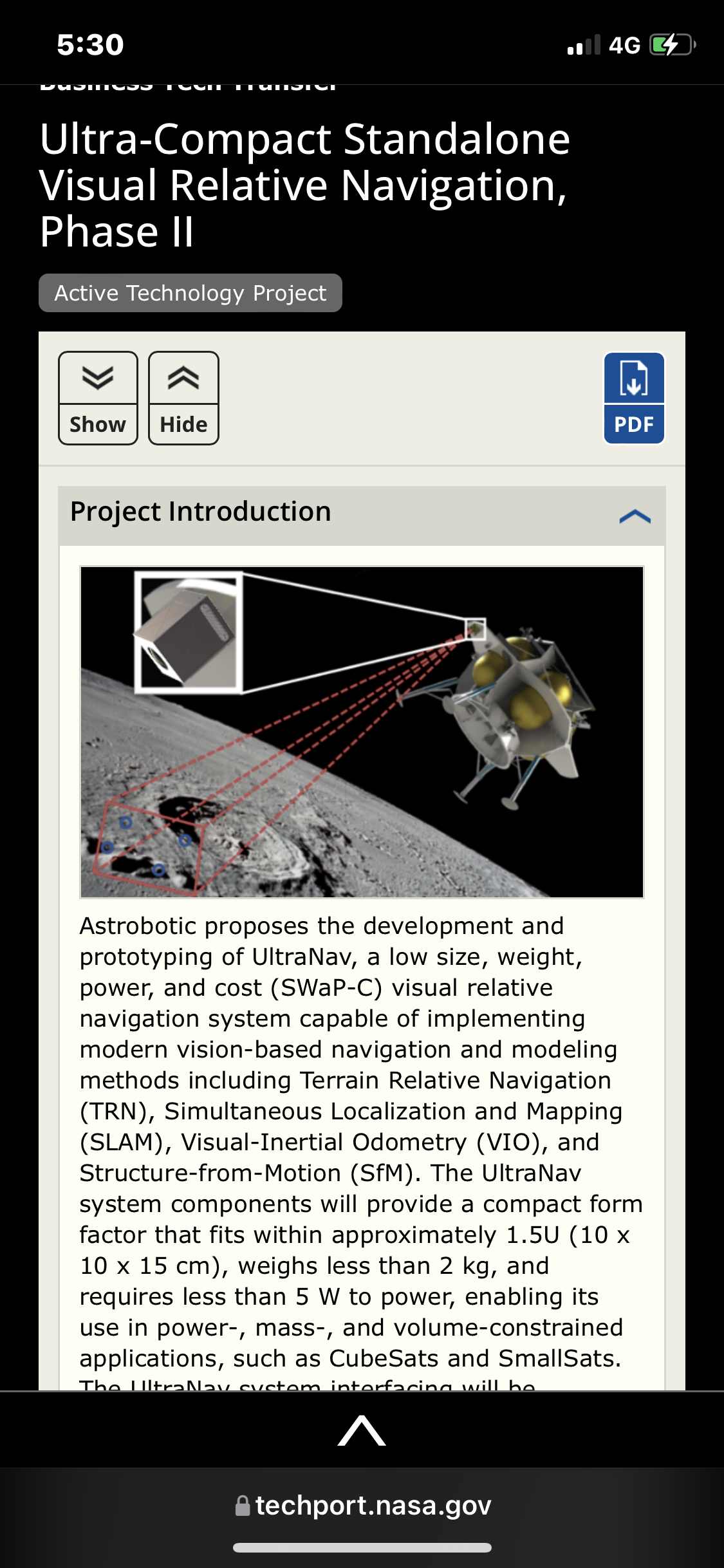
 A efficient radiation-tolerant System-on-Chip (SoC) will be integrated into a larger system that includes
A efficient radiation-tolerant System-on-Chip (SoC) will be integrated into a larger system that includes
ReRAM
ReRAM has the benefit of inherently radiation immunity.
D
Deleted member 118
Guest
Think it makes sense nowReRAM has the benefit of inherently radiation immunity.
D
Deleted member 118
Guest
Very interesting that nasa are pushing to develope(finalise) a VOC device and I wonder if they are trying to utilise Akida into it.
Now that would be something else and maybe that’s where a few of our chips have gone.
Respiratory viral infection can sometimes lead to serious, possibly life threatening complications. Highly contagious respiratory diseases cause significant disruptions to social and economic systems if spread is uncontrolled. Therefore, the rapid and precise identification of viral infection before entering crowded or vulnerable areas is essential for suppressing their transmission effectively. Additionally, a device that is reconfigurable to address the next pandemic is highly desired. Screening for infection via exhaled breath analysis could provide a quick and simple method to find infectious carriers. This breath analyzer conceptualizes a rapid scanning device enabling the user to determine the presence of viral infection in an exhaled breath through analyzing volatile organic compounds (VOCs) concentrations.N5 Sensors will technically evaluate the feasibility of volatile organic compounds (VOCs) sensors for realizing Rapid Infection Screening via Exhalation (RISE) in a breathalyzer able to identify respiratory virus infected individuals, suitable for mass-testing scenarios.The proposed survey is expected to provide the guidance how to devise an integrated sensor system for actualizing initial screening at key check points. The evaluation will be accomplished by performing market survey, research level survey, and receiving consulting from breath analyzer pioneering companies for 1) Breath analyzer platform2) VOC gas sensors and 3) Machine learning algorithm. The survey will be progressed within stepwise assessment from initial database search, article screening and selection, to quality assessment and assortment. A comprehensive final report will be provided in which our findings and research strategy for Phase II are presented.
Triton Systems, Inc. will identify, design, and develop three noninvasive diagnostic tools to screen breath for the presence of communicable respiratory viral infections. The proposed screening technologies will be based on low-cost, high-throughput sensing modalities capable of detecting unique signatures of viral pathogens. After conducting a thorough and technical review of available targets, including volatile organic contaminants (VOCs), and suitable sensing platforms, Triton will develop sensor components into an integrated system with minimal form factor for use as personal health monitors or at travel checkpoints in highly trafficked areas. The proposed sensors will be easy to administer and widely deployable to maximize their benefit during seasonal epidemics and global pandemics involving communicable respiratory viral infections. Emphasis will be placed on an inexpensive platform with superlative sensitivity, selectivity, stability, and throughput. Wireless communication capabilities will enable the presentation and recording of the screening results in under five minutes at the site of use. Combined, the sensor components will be a widely deployable platform for detecting viral respiratory agents with pandemic potential, enhancing public health emergency readiness, and improving the transportation security infrastructure in the United States.
The COVID-19 pandemic is an example of human vulnerability to new communicable respiratory viral infections. Currently, most viral respiratory infections in humans are detected by sensing the presence of the pathogen's genetic material or proteins (i.e., antigens) in bodily fluids. Polymerase chain reaction (PCR)-based methods are the most commonly used to detect a pathogen's genetic material. Although samples can be collected outside the lab, it requires specialized laboratories and skilled technicians to collect samples, perform the tests, and analyze results. Furthermore, these tests requires hours to days to process and provide results. Additionally, their sampling methods are generally invasive. Accelerating the development of new, near real time, inexpensive, user-friendly, non-invasive, accurate, and sensitive detection technologies will contribute significantly to the national and worldwide efforts to curb communicable respiratory viral infections, like the COVID-19 pandemic. During Phase I, Lynntech will use its extensive expertise in portable chemical and biochemical sensor development (including sensors for VOC detection) to select portable, fast, reliable sensors/detectors that could be used to detect VOC markers in exhaled breath and that are associated with infectious agents. During Phase II, Lynntech will develop prototypes of the candidate approach and conduct tests to demonstrate the device's capability in the detection of VOC markers of a viral infection.
Last edited: 12
Now that would be something else and maybe that’s where a few of our chips have gone.
Respiratory viral infection can sometimes lead to serious, possibly life threatening complications. Highly contagious respiratory diseases cause significant disruptions to social and economic systems if spread is uncontrolled. Therefore, the rapid and precise identification of viral infection before entering crowded or vulnerable areas is essential for suppressing their transmission effectively. Additionally, a device that is reconfigurable to address the next pandemic is highly desired. Screening for infection via exhaled breath analysis could provide a quick and simple method to find infectious carriers. This breath analyzer conceptualizes a rapid scanning device enabling the user to determine the presence of viral infection in an exhaled breath through analyzing volatile organic compounds (VOCs) concentrations.N5 Sensors will technically evaluate the feasibility of volatile organic compounds (VOCs) sensors for realizing Rapid Infection Screening via Exhalation (RISE) in a breathalyzer able to identify respiratory virus infected individuals, suitable for mass-testing scenarios.The proposed survey is expected to provide the guidance how to devise an integrated sensor system for actualizing initial screening at key check points. The evaluation will be accomplished by performing market survey, research level survey, and receiving consulting from breath analyzer pioneering companies for 1) Breath analyzer platform2) VOC gas sensors and 3) Machine learning algorithm. The survey will be progressed within stepwise assessment from initial database search, article screening and selection, to quality assessment and assortment. A comprehensive final report will be provided in which our findings and research strategy for Phase II are presented.
Triton Systems, Inc. will identify, design, and develop three noninvasive diagnostic tools to screen breath for the presence of communicable respiratory viral infections. The proposed screening technologies will be based on low-cost, high-throughput sensing modalities capable of detecting unique signatures of viral pathogens. After conducting a thorough and technical review of available targets, including volatile organic contaminants (VOCs), and suitable sensing platforms, Triton will develop sensor components into an integrated system with minimal form factor for use as personal health monitors or at travel checkpoints in highly trafficked areas. The proposed sensors will be easy to administer and widely deployable to maximize their benefit during seasonal epidemics and global pandemics involving communicable respiratory viral infections. Emphasis will be placed on an inexpensive platform with superlative sensitivity, selectivity, stability, and throughput. Wireless communication capabilities will enable the presentation and recording of the screening results in under five minutes at the site of use. Combined, the sensor components will be a widely deployable platform for detecting viral respiratory agents with pandemic potential, enhancing public health emergency readiness, and improving the transportation security infrastructure in the United States.
The COVID-19 pandemic is an example of human vulnerability to new communicable respiratory viral infections. Currently, most viral respiratory infections in humans are detected by sensing the presence of the pathogen's genetic material or proteins (i.e., antigens) in bodily fluids. Polymerase chain reaction (PCR)-based methods are the most commonly used to detect a pathogen's genetic material. Although samples can be collected outside the lab, it requires specialized laboratories and skilled technicians to collect samples, perform the tests, and analyze results. Furthermore, these tests requires hours to days to process and provide results. Additionally, their sampling methods are generally invasive. Accelerating the development of new, near real time, inexpensive, user-friendly, non-invasive, accurate, and sensitive detection technologies will contribute significantly to the national and worldwide efforts to curb communicable respiratory viral infections, like the COVID-19 pandemic. During Phase I, Lynntech will use its extensive expertise in portable chemical and biochemical sensor development (including sensors for VOC detection) to select portable, fast, reliable sensors/detectors that could be used to detect VOC markers in exhaled breath and that are associated with infectious agents. During Phase II, Lynntech will develop prototypes of the candidate approach and conduct tests to demonstrate the device's capability in the detection of VOC markers of a viral infection.
Last edited: 12
D
Deleted member 118
Guest
Thoughts @Fact Finder
broader impact/commercial potential of this Small Business Innovation Research (SBIR) Phase II project is to enable energy efficient smart internet of things (IoT) devices capable of running a neural network locally. The proposed energy-efficient neural network accelerator solution uses circuit architecture that allows for chips with a small area, a key enabler for cost-effective adoption and inclusion in space-constrained systems such as mobile devices. The solution is energy-efficient compared to the existing digital logic-based accelerator solutions, which will enable edge implementation for systems with power constraints. The manufacturing process is fully scalable in advanced standard logic processes at almost all manufacturing foundries, thus allowing for widespread adoption of the architecture. The outcome of this project will be an energy-efficient system on a chip (SoC) solution that offers artificial intelligence integration in smart IoT devices without cloud access, while enabling security and privacy enhancements. This Small Business Innovation Research (SBIR) Phase II project seeks to further develop an energy efficient analog circuit topology and variation tolerable system solution. To enable analog compute-in-memory architecture based neural network accelerator solution in an advanced semiconductor process technology, significant design challenges need to be solved with reduced supply voltage and noise margin. Along with the newly proposed area efficient and performance efficient analog compute-in-memory architecture solution, the logic compatible non-volatile neural network accelerator intellectual property core will be designed, fabricated, and validated in the advanced process technology through the project. Once verified successfully from the fabricated silicon in this project, the proposed neural network IP will be ready to be integrated as a key building block of future artificial intelligence systems on a chip and enable energy-efficient smart edge IoT devices. This award reflects NSF's statutory mission and has been deemed worthy of support through evaluation using the Foundation's intellectual merit and broader impacts review criteria.
broader impact/commercial potential of this Small Business Innovation Research (SBIR) Phase II project is to enable energy efficient smart internet of things (IoT) devices capable of running a neural network locally. The proposed energy-efficient neural network accelerator solution uses circuit architecture that allows for chips with a small area, a key enabler for cost-effective adoption and inclusion in space-constrained systems such as mobile devices. The solution is energy-efficient compared to the existing digital logic-based accelerator solutions, which will enable edge implementation for systems with power constraints. The manufacturing process is fully scalable in advanced standard logic processes at almost all manufacturing foundries, thus allowing for widespread adoption of the architecture. The outcome of this project will be an energy-efficient system on a chip (SoC) solution that offers artificial intelligence integration in smart IoT devices without cloud access, while enabling security and privacy enhancements. This Small Business Innovation Research (SBIR) Phase II project seeks to further develop an energy efficient analog circuit topology and variation tolerable system solution. To enable analog compute-in-memory architecture based neural network accelerator solution in an advanced semiconductor process technology, significant design challenges need to be solved with reduced supply voltage and noise margin. Along with the newly proposed area efficient and performance efficient analog compute-in-memory architecture solution, the logic compatible non-volatile neural network accelerator intellectual property core will be designed, fabricated, and validated in the advanced process technology through the project. Once verified successfully from the fabricated silicon in this project, the proposed neural network IP will be ready to be integrated as a key building block of future artificial intelligence systems on a chip and enable energy-efficient smart edge IoT devices. This award reflects NSF's statutory mission and has been deemed worthy of support through evaluation using the Foundation's intellectual merit and broader impacts review criteria.
Last edited by a moderator:
D
Deleted member 118
Guest
Another one for the book
 www.sbir.gov
www.sbir.gov
In order to meet the Navy’s need for a spiking neural network testing platform, ChromoLogic proposes to develop a Spiking Neural Network Modeler (SpiNNMo) capable of simulating a variety of neuromorphic hardware platforms. SpiNNMo will allow the Navy to test the performance of new spiking neural network architectures and hardware technologies in their RF signal processing pipelines. SpiNNMo simulates errors inherent in analog computational circuits at the neuron-level so that a single model can simulate the behavior of a wide range of underlying hardware without the computational cost of simulating individual circuit elements.
Spiking Neural Network Modeler (SpiNNMo) | SBIR.gov
In order to meet the Navy’s need for a spiking neural network testing platform, ChromoLogic proposes to develop a Spiking Neural Network Modeler (SpiNNMo) capable of simulating a variety of neuromorphic hardware platforms. SpiNNMo will allow the Navy to test the performance of new spiking neural network architectures and hardware technologies in their RF signal processing pipelines. SpiNNMo simulates errors inherent in analog computational circuits at the neuron-level so that a single model can simulate the behavior of a wide range of underlying hardware without the computational cost of simulating individual circuit elements.
D
Deleted member 118
Guest
This looks interesting
The broader impact/commercial potential of this Small Technology Transfer Research (STTR) Phase I project lays in its ability to enable reconfigurable hardware acceleration in the Internet-of-Things (IoT). Users under constrained power at the edge will be able to choose a new solution that can bring acceleration, and enable datacenter like capabilities, and benefit from the IoT's long-sought promise. Using Resistive Random-Access Memory (RRAM) to develop a next-generation Field Programmable Gate Array (FPGA), increasing performance while reducing energy consumption at the sensor node level, is possible. As already experienced in our data driven world, it is critical that we improve our computing capabilities at the edge in order to gain in responsiveness and increase our energy efficiency. With evermore data and performance requirements to deliver on the consumer demands, innovative uses of emerging memories are showing great promise and providing capabilities that will help fulfill the IoT's potential. If fulfilled, this technology has the potential to enable a whole set of data driven applications at the edge, such as low-energy image recognition and learning in drones to operate longer and more effectively or long-lasting medical implants with leading data aggregation and reactiveness. This Small Technology Transfer Research (STTR) Phase I project will aim to commercialize a patented technology to realize a ultra-low-power Field Programmable Gate Array (FPGA) based on Resistive Random-Access Memory (RRAM). To handle the data explosion in Internet of Things (IoT) network, the industry is moving towards increasing intelligent analysis capability for single IoT devices. Tight power budget has become a critical road block: high-end solutions, such as multicore CPUs, GPUs, can provide enough computing capability but fail to meet the power budget, while low-power commercial products, such as micro-controllers and low-power FPGAs, can satisfy power constraints but hardly follow the increasing complexity in data analysis algorithms. This project aims to develop a ultra-low-power FPGA that can offer high-performance data analysis capability under IoT-level power limits. This project will prototype a FPGA chip built around an innovative RRAM-based routing multiplexer design. We will also release an associated software tool suite to support the implementation of customer's applications on the technology. Compared to existing commercial solutions, the proposed FPGA product is expected to demonstrate similar computing capability as high-end FPGA solutions while satisfying an IoT power budget (<1W). This award reflects NSF's statutory mission and has been deemed worthy of support through evaluation using the Foundation's intellectual merit and broader impacts review criteria.
The broader impact/commercial potential of this Small Technology Transfer Research (STTR) Phase I project lays in its ability to enable reconfigurable hardware acceleration in the Internet-of-Things (IoT). Users under constrained power at the edge will be able to choose a new solution that can bring acceleration, and enable datacenter like capabilities, and benefit from the IoT's long-sought promise. Using Resistive Random-Access Memory (RRAM) to develop a next-generation Field Programmable Gate Array (FPGA), increasing performance while reducing energy consumption at the sensor node level, is possible. As already experienced in our data driven world, it is critical that we improve our computing capabilities at the edge in order to gain in responsiveness and increase our energy efficiency. With evermore data and performance requirements to deliver on the consumer demands, innovative uses of emerging memories are showing great promise and providing capabilities that will help fulfill the IoT's potential. If fulfilled, this technology has the potential to enable a whole set of data driven applications at the edge, such as low-energy image recognition and learning in drones to operate longer and more effectively or long-lasting medical implants with leading data aggregation and reactiveness. This Small Technology Transfer Research (STTR) Phase I project will aim to commercialize a patented technology to realize a ultra-low-power Field Programmable Gate Array (FPGA) based on Resistive Random-Access Memory (RRAM). To handle the data explosion in Internet of Things (IoT) network, the industry is moving towards increasing intelligent analysis capability for single IoT devices. Tight power budget has become a critical road block: high-end solutions, such as multicore CPUs, GPUs, can provide enough computing capability but fail to meet the power budget, while low-power commercial products, such as micro-controllers and low-power FPGAs, can satisfy power constraints but hardly follow the increasing complexity in data analysis algorithms. This project aims to develop a ultra-low-power FPGA that can offer high-performance data analysis capability under IoT-level power limits. This project will prototype a FPGA chip built around an innovative RRAM-based routing multiplexer design. We will also release an associated software tool suite to support the implementation of customer's applications on the technology. Compared to existing commercial solutions, the proposed FPGA product is expected to demonstrate similar computing capability as high-end FPGA solutions while satisfying an IoT power budget (<1W). This award reflects NSF's statutory mission and has been deemed worthy of support through evaluation using the Foundation's intellectual merit and broader impacts review criteria.
Similar threads
- Replies
- 0
- Views
- 4K
- Replies
- 9
- Views
- 6K
- Replies
- 0
- Views
- 3K